This spring holiday I took the recently published Study on Corruption in the Healthcare Sector with me as holiday reading and that proved a good choice. The 332 pages long study was commissioned by the European Commission (EC) Directorate-General Home Affairs and involved cooperation between Ecorys, the European Healthcare Fraud & Corruption Network (EHFCN) and individual country correspondents in 28 European Union Member States.
This spring holiday I took the recently published Study on Corruption in the Healthcare Sector with me as holiday reading and that proved a good choice. The 332 pages long study was commissioned by the European Commission (EC) Directorate-General Home Affairs and involved cooperation between Ecorys, the European Healthcare Fraud & Corruption Network (EHFCN) and individual country correspondents in 28 European Union Member States.
It serves as input to the first EU Anti-Corruption Report, which is part of the overall anti-corruption strategy initiated by the European Commission in 2011.
Objectives
The study’s objectives are to
- enable a better understanding of the extent, nature and impact of corrupt practices in the healthcare sector across the EU; and
- assess the capacity of the Member States to prevent and control corruption within the healthcare system and the effectiveness of these measures in practice.
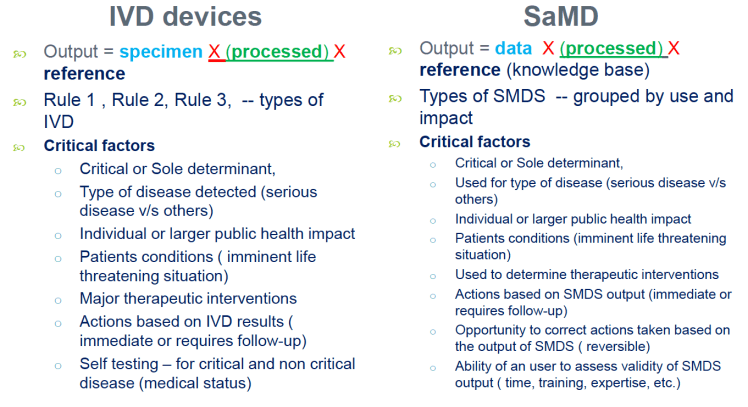
The focus lies on three areas of healthcare: (i) medical service delivery (various forms of informal payments); (ii) procurement and certification of medical devices; and (iii) procurement and authorisation of medicinal products.
Useful for compliance counsel
As I have been able to determine for myself, this focus makes the study report very useful tool for compliance counsel and/or staff in a larger medical devices (or any healthcare) company that does business in Europe and that has to match business compliance obligations in multiple areas and under different statutes with international dimensions, like the US FCPA and UK Anti-Bribery Act.
The study uses the term ‘corruption’ quite loosely. In the study this term covers a plethora of practices that a company may be faced with (and possibly engage in) when doing business in the healthcare sector in the EU, like misuse of influence, bribery and unethical marketing practices. It gives a nice typology of modalities corruption in healthcare, lists a large number (86) of case studies and in the process does a great job of describing compliance risks in the respective member states (although the writers of the report clearly received more input from some member states than from others).
What industry is not to allowed to give, doctors shouldn’t be allowed to ask
The study does a good job of describing the dilemma of lack of funds for training and (continued) education for physicians and the widely held expectation that industry will pony up the required funds one way or the other without being allowed to expect anything special treatment or consideration in return because that would be unethical.
The study clearly describes the – in my view – often neglected / overlooked role of the medical profession and healthcare institution management in initiation and perpetuation of unethical practices. While the study focuses on prosecution of individual doctors, it does not mention very positive developments like in the Netherlands (the study often refers to the Netherlands) where the associations of hospitals and doctors have also adhered to the self regulatory GMH Code. This way the loop has been closed – albeit with some firm coercion from the Ministry of Health to make the medical profession take their responsibility in this respect. This kind of closing the loop is – in my view – the only way to reduce unethical behavior in the healthcare market. You may say that I suffer from professional cynicism, but as long as the demand side still can expect behavior that is deemed unethical when exhibited on the supply side, unethical supply and unethical demand will always find a way to connect and it is in practice pretty difficult to determine what side initiated the unethical practice.
Certification fraud
The report also has a section discussing certification fraud / corruption with respect to medical devices, which is interesting in the light of recent accusations addressed to the notified bodies following the PIP breast implants and metal-on-metal hip implant cases that they are not well equipped to be gatekeeper for medical devices market access that played an important part in the medical devices directives revision. I must say I was a bit disappointed by the discussion in the report, which was somewhat thin and the conclusion seems to suggest issues the report does not support. The study report spends some time defining possible risks, like the alleged revolving door between industry and notified bodies / regulators, and describes developments in the new notified bodies code of conduct by Team NB. However, it does not discuss to a single concrete case and suffices to state that:
“Within the areas of pharmaceuticals and medicals devices very few cases have reported concerning the specific issues of authorization and certification. This may be the result of the rather technical nature of these processes that are only known in detail by a small number of people in each country. This may have influenced the identification of issues and nature of interview respondents in several countries.”
It may have also been that just almost no cases exist in this respect, which is the study seems to want to deliberately overlook as another plausible conclusion.
Procurement
Personally I was very happy with the good work on compliance issues in procurement and the detailed topology of all the ways that procurement processes can be unduly influenced both from the outside and from the inside. The study gives a balanced overview of things by also discussing the doctors’ and healthcare institutions’ role in procurement compliance issues. If your company sells via tenders in the EU, this is a section of the study you should definitely read.
Local differences
The study describes the different ways that member states are dealing with the compliance issues in healthcare, and proves what everyone in EU compliance matters already knows: there is no one size fits all solution that works in the same way in every member state. The issues are also not of the same type and prevalence in each member state.
The study goes into a lot of detail when describing the different systems that member states use: self-regulatory rules as well as criminal, administrative and civil law. It shows how some approaches worked and how some failed. Interestingly it also tries to show how the failed approaches failed because they failed to provide the right (dis)incentives, with unintended consequences as result.
Recommendations
As a conclusion the study recommends that
“to address drivers of corruption that prevail in all EU MSs, EU-wide policies are needed. At the EU level it is recommended to a) set clear and effectively enforced general anti-corruption rules (e.g. UK Bribery Act and US Foreign Corrupt Practices Act), b) introduce independent and effective judicial follow up on corruption cases, and c) implement sound and transparent general procurement systems. General public procurement policies should also apply for the healthcare sector.
Another aspect that can be addressed at EU level concerns self-regulation, for example through a Code of Conduct or Code of Ethics. Industry organisations at the EU level, such as EUCOMED and EFPIA, have these already in place for their members. Self-regulation should also be organised at a national level. The good practices in the Netherlands and the United Kingdom illustrate that conditioned self-regulation can be an effective way of regulation a sector. It is recommended to find the right balance between formal regulation (legislation) and self-regulation and clearly define how the two function in parallel and complement each other.”
That is easier said than done however. The European Commission does not have any formal tools to make groups of companies work together and in practice the different groups in medicinal products and medical devices do not always cooperate smoothly. Cooperation in self-regulation will need to be a bottom-up approach, like the process of convergence that EDMA and Eucomed have started under the MedTech Europe name. But we have the Dutch on the case now trying to see if they can get an EU level policy started by gathering support from the other member states for addressing HCP-industry relations in the draft medical devices regulation. So far they have not gotten much traction, which shows that the member states are divided on this.
At national level the study recommends effective sanctions, transparency and active reporting by media, patient groups and individuals. Not the most surprising of conclusions perhaps, but that is often the case with empirically answered questions that do not falsify the initial intuitive answer to the research hypothesis.
Useful stuff in the annexes
The annexes contain useful very useful information for companies, such as
- case studies;
- overview of perceptions of corruption in the member states;
- country reports per member state, with very convenient descriptions of the healthcare systems (including reimbursement systems) and compliance phenomena observed; and
- a corruption risk checklist.
In other words, a wealth of useful information that you would otherwise pay a consultant a lot of money for. Use it at your advantage.
Want to know more?
Aline Lautenberg, the General Counsel – Director Legal & Compliance of Eucomed puts the study in the context of other developments in her recent blog post here. The report and other developments will be discussed at the Global MedTech Compliance Conference in Barcelona on 20-22 May. See you there!